COPD - Emphysema Treatment
What is the Zephyr® Valve:
The first FDA-approved, minimally-invasive device available in the U.S. for treating patients with severe emphysema.
Who is the Zephyr® Valve for?
Severe emphysema patients who consistently feel short of breath despite using COPD medications and/or oxygen.
What are the Benefits of Zephyr® Valves?
Patients report being able to take full breaths immediately after the procedure and within a few days are back to doing everyday tasks with ease. In clinical studies patients treated with Zephyr® Valves have been shown to1:
- Breathe easier
- Be more active and energetic
- Be less short of breath
- Enjoy a significantly improved quality of life compared to untreated patients.
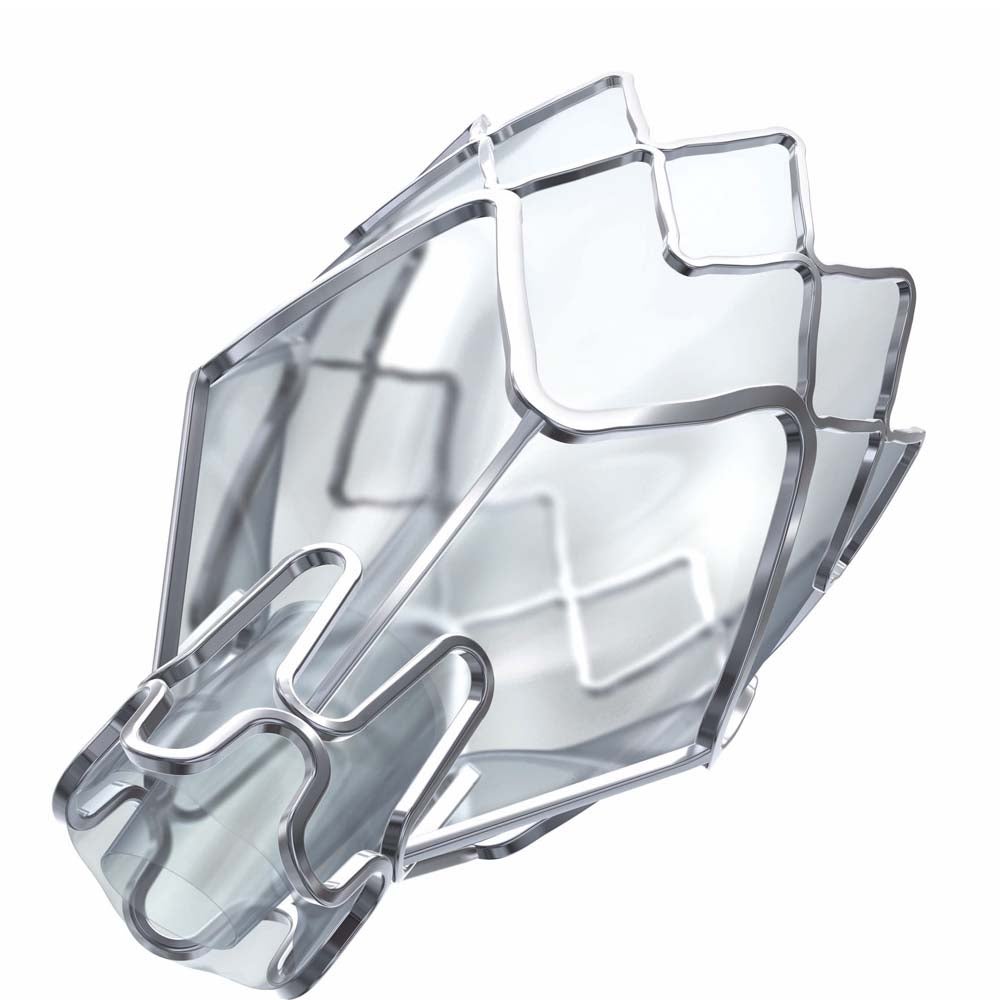
How does the Procedure Work?
The one-time procedure is done during a bronchoscopy that requires no cutting or incisions. During the procedure, on average 4 tiny valves are placed in the airways to block off the diseased parts of the lungs. The valves reduce hyperinflation, preventing air from being trapped in the diseased area of the lung and allowing healthier parts of the lung to take in more air. This results in patients being able to breathe easier. Patients treated have reported immediate relief.
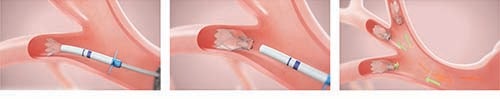
What can Patients Expect?
A typical Zephyr® valve procedure looks like this:
- The doctor will give you medicine to make you sleepy.
- A small tube with a camera, called a bronchoscope, will be inserted into your lungs through your nose or mouth.
- During the procedure on average the doctor will place 4 Zephyr Valves in the airways.
- You will stay in the hospital for approximately 3 nights for observation.
- After the procedure, you will continue to use the medicines that your doctor has prescribed for your condition.
How do I schedule a consultation to see if I am a candidate for the Zephyr Valves?
Please contact 954-491-8981.
For more information about the technology, go to MyLungsMyLife.com.
Complications of the Zephyr Endobronchial Valve treatment can include but are not limited to pneumothorax, worsening of COPD symptoms, hemoptysis, pneumonia, dyspnea and, in rare cases, death.
1 Criner G et al AM J Resp Crit Care Med 2018, Published on 22-May-2018 as 10.1164/rccm.201803-0590OC
Brief Statement: The Pulmonx Zephyr® Endobronchial Valves are implantable bronchial valves indicated for the bronchoscopic treatment of adult patients with hyperinflation associated with severe emphysema in regions of the lung that have little to no collateral ventilation. The Zephyr Valve is contraindicated for: Patients for whom bronchoscopic procedures are contraindicated; Patients with evidence of active pulmonary infection; Patients with known allergies to Nitinol (nickel-titanium) or its constituent metals (nickel or titanium); Patients with known allergies to silicone; Patients who have not quit smoking; Patients with large bullae encompassing greater than 30% of either lung. Use is restricted to a trained physician. Prior to use, please reference the Zephyr Endobronchial Valve System Instructions for more information on indications, contraindications, warnings, all precautions, and adverse events.
Caution: Federal law restricts this device to sale by or on the order of a physician.
©2019 Pulmonx Corporation or its affiliates. All rights reserved. All trademarks are property of their respective owners.
P0773EN_A May 2019
